eblThe alkaline batteries we use today made their debut in 1959. By the mid-1980s, they had surpassed zinc-carbon batteries as the most popular battery chemistries. Since their introduction billions [probably trillions] of units have been consumed in every part of the world. As far back as 2009, there were an estimated 50 billion alkaline battery units in circulation, amounting to an average of between 7 to 8 units per individual each year.

Today, the alkaline battery market is a multi-billion-dollar industry projected to grow further in the future. This success, even in the face of competition from other battery chemistries is due to some distinct properties of the alkaline chemistry.
What is an alkaline battery?
Though a number of battery chemistries are designed to work with alkaline electrolytes, alkaline batteries usually refer to the alkaline zinc-manganese dioxide primary cells. In an alkaline battery, the positive electrode or cathode contains manganese (IV) oxide while the negative electrode or anode contains zinc.
Initial alkaline batteries were designed as primary batteries meant to be disposed of after a single use. However, advancements in technology have led to the development of rechargeable alkaline chemistries.
Alkaline Battery Electrochemistry Explained
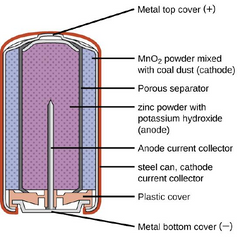
An alkaline battery produces power via a couple of chemical reactions at its positive and negative electrodes. In the cathode, manganese (IV) oxide is transformed into manganese (III) oxide and hydroxyl ions. These hydroxyl ions then react with zinc in the anode to release power-generating electrons.
Here is a simple equation for an alkaline cell reaction:
Zn + 2MnO2 + H2O → ZnO +2MnOOH
In the above reaction, it can be seen that H2O (water) is used up. Subsequently, hydroxyl ions (OH-) are created by the MnO2 cathode as shown in the reaction below:
2MnO2 + 2H2O+ 2 e → 2MnOOH + 2OH-
Also, the anode simultaneously uses up hydroxyl ions while creating water:
Zn + 2OH- →ZnO +H2O + 2e
Finally, the resultant electrons (e) from the reaction yield the power used for different kinds of gadgets or appliances.
Alkaline battery sizes
The most common alkaline battery sizes include:
- AAAA cells
- AAA cells
- AA cells
- D cells
- C cells
- N cells
- 9V cells
- Button cells
Some Common Alkaline Battery Applications
- Flashlights🔦
- Clocks⏰
- Electronic thermometers
- Game controllers🕹
- Cameras📷
- Digital voltmeters
- Remote controls
- Portable radios📻
- Door locks
- Laser pointers
- Toys🧸
- Mobile scanners
- Electric toothbrushes
- Portable transmitters
- Electric toothbrushes, etc
Factors behind the popularity of alkaline batteries
As noted above, the alkaline battery is now the most popular battery around after surpassing the zinc-carbon chemistry many years ago. Here are some of the reasons why there is still a huge market for alkaline batteries even with the emergence of newer chemistries such as NiCD, NiMH, and Lithium-ion.
☘High Energy Density
Users who desire longevity will find alkaline batteries very useful. This is because alkaline batteries have the highest energy density when compared to other chemistries. For instance, they have double [or more] the energy density of NIMH and zinc-carbon batteries.
☘Cost-effective
Alkaline batteries are often the most affordable batteries you’ll get on the market. This can be attributed to lower production costs and comparatively less expensive chemical constituents.
☘Lower Temperatures
With alkaline batteries, incidents of device damage or sub-optimal performance due to overheating will not arise. Reason? Alkaline chemistries generate less heat while in operation.
☘Longer Shelf Life
Shelf life is the length of time a disposable alkaline battery will retain its charge unused or in the case of rechargeable batteries, how long it will stay unused [without significant deterioration] before needing to be charged. A battery’s shelf life can be influenced by certain factors such as the battery’s design, chemical composition, storage temperature, and humidity.
Non-rechargeable alkaline batteries have a shelf life of as much as 10 years which is among the highest in the industry.
☘Design Improvements
The need for more convenience has recently led to improvements in battery packaging techniques. Previously, alkaline batteries were designed with complex sealing systems including thick steel outer cases and end caps. With time, these have gradually given way to a new unique thinner packaging and more efficient seals. This has translated to extra room for battery components and hence increased performance capacity.
☘Suitable for Potting
Because they are not characterized by internal temperature changes and are not prone to gassing when in active use, alkaline batteries are very suitable for potting. Potting is a process whereby an enclosure is filled with a chemical compound in order to boost its capacity to resist shock and vibration while also protecting things like solvents, corrosion, and moisture.
☘Available in Different Sizes
As seen above, alkaline batteries are designed in different sizes. This makes them convenient to move about with and easy to use in different kinds of household, office, and factory devices.
☘Can be Connected Serially
It is possible to connect a series of alkaline batteries in order to generate arrays of higher voltage. They can also be arranged in different configurations to serve a broad range of purposes.
☘Easier to Dispose of/Environmentally Safer
The major chemical constituents of alkaline batteries are zinc, manganese, and steel, all of which are less toxic and hence do not constitute any significant threats either to human or environmental health. Moreover, the removal of mercury from the battery’s electrochemistry since the 1990s has made it possible to dispose of spent alkaline batteries like any other household waste in most jurisdictions.
☘Leak-resistant
Some usually associate alkaline batteries with rupture and leakage. But this is not the case if you opt for quality alkaline batteries from a renowned producer especially since there are several products in the market that are not as efficient as others.
So Which alkaline battery is best for use?
With a lot of competing alkaline battery brands out there, consumers will definitely not find it easy to make a choice. However, one excellent alkaline battery product you can trust is when it comes to anti-leakage protection, safety, maximum shelf life, longevity and other qualities desired in an alkaline battery is EBL.
EBL high-performance yet very affordable alkaline batteries have been manufactured with premium raw materials and high-density cell technology that will give your devices enough power while lasting longer, enabling your various devices to perform optimally as well as save you money. If you wish for the best service from an alkaline battery, then EBL should be your choice! Visit the our website today for more information.










































Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.